Budesonide is an inhaled corticosteroid (ICS) globally marketed today as a generic Dry Powder Inhaler (DPI) having been originally developed by AstraZeneca for use in varying formulations through their Turbuhaler device.
Using the Budesonide Turbuhaler as the reference listed drug ( RLD), Knightpoint with their partners, Intertek, developed a formulation to compare through testing on Aerodynamic Particle Size Distribution (APSD) by NGI and Dose Uniformity.
This formulation was delivered through a prototype Mirai device engine using the developed airflow pathways and airflow resistance.
The results obtained were extremely encouraging with a good APSD profile match with the RLD on NGI testing.The delivered dose uniformity was also encouraging.
We have also delivered comparable performance with the RLD for another ICS in Fluticasone Propionate.
Using our partners and our own formulation expertise Knightpoint are confident we can help you meet your DPI needs.
Formulations
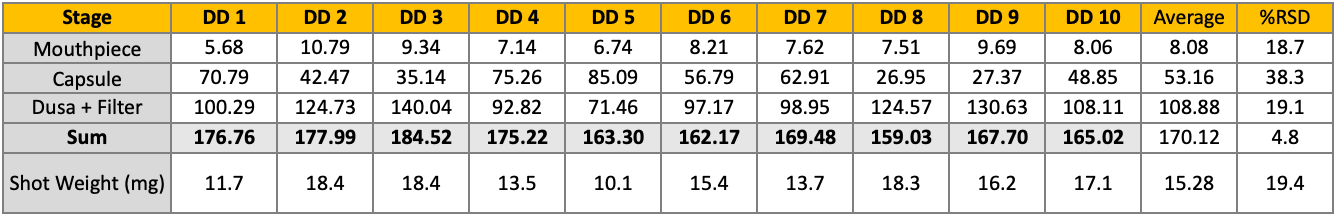
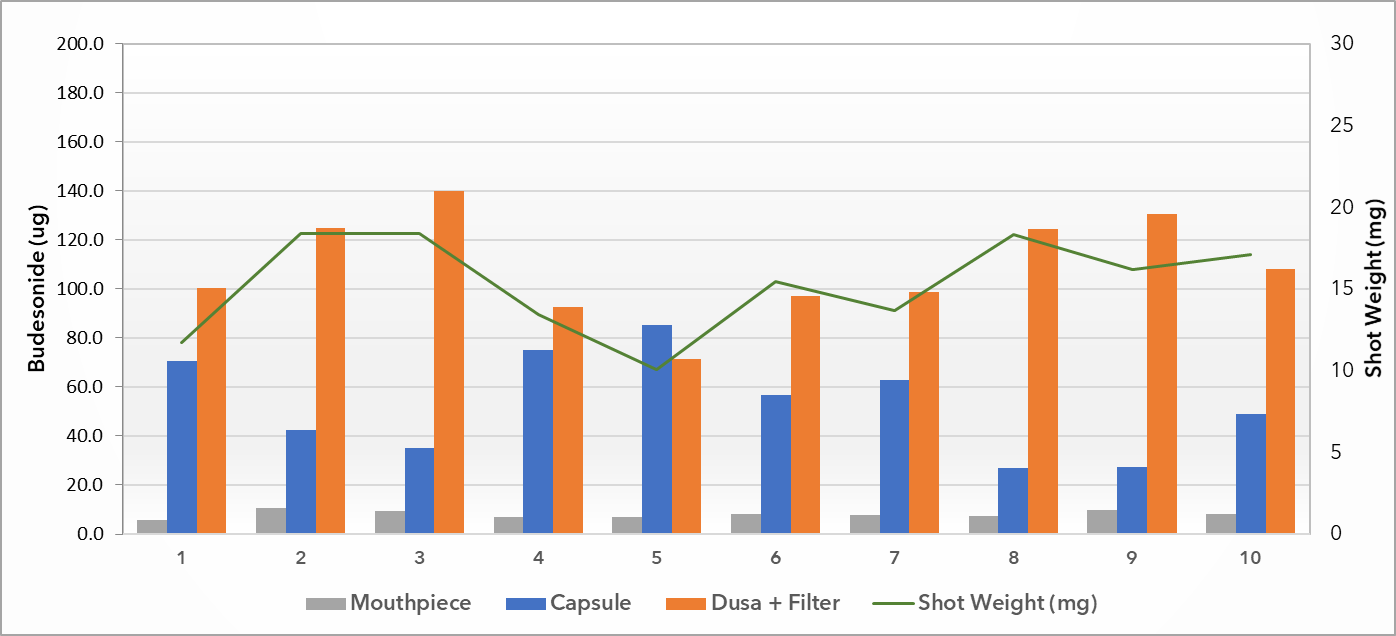
APSD for Budesonide comparison with RLD
Similar profile exhibited to RLD
Less than 5ug difference in sum of stages 3-5
Less than 12ug difference between throat to stage 8
Minor differences accounted for by early stage device development